Advancing Cancer Research with Tumoroid Technologies: A Convergence of Microfluidics, Organoids, and Immuno-Oncology
Introduction
Cancer research has undergone a paradigm shift with the emergence of in vitro tumor models that closely mimic in vivo conditions. Among these, Tumoroid-on-a-Chip technology, Organoid-Tumoroid Hybrid Models, Tumoroid-Derived Extracellular Vesicles (EVs), and Integration with Immuno-Oncology Models are revolutionizing cancer biology, drug development, and personalized medicine. This blog explores these cutting-edge technologies, their applications, and their potential to transform cancer therapy.
1. Tumoroid-on-a-Chip Technology: Mimicking the Tumor Microenvironment (TME)
1.1 What is Tumoroid-on-a-Chip?
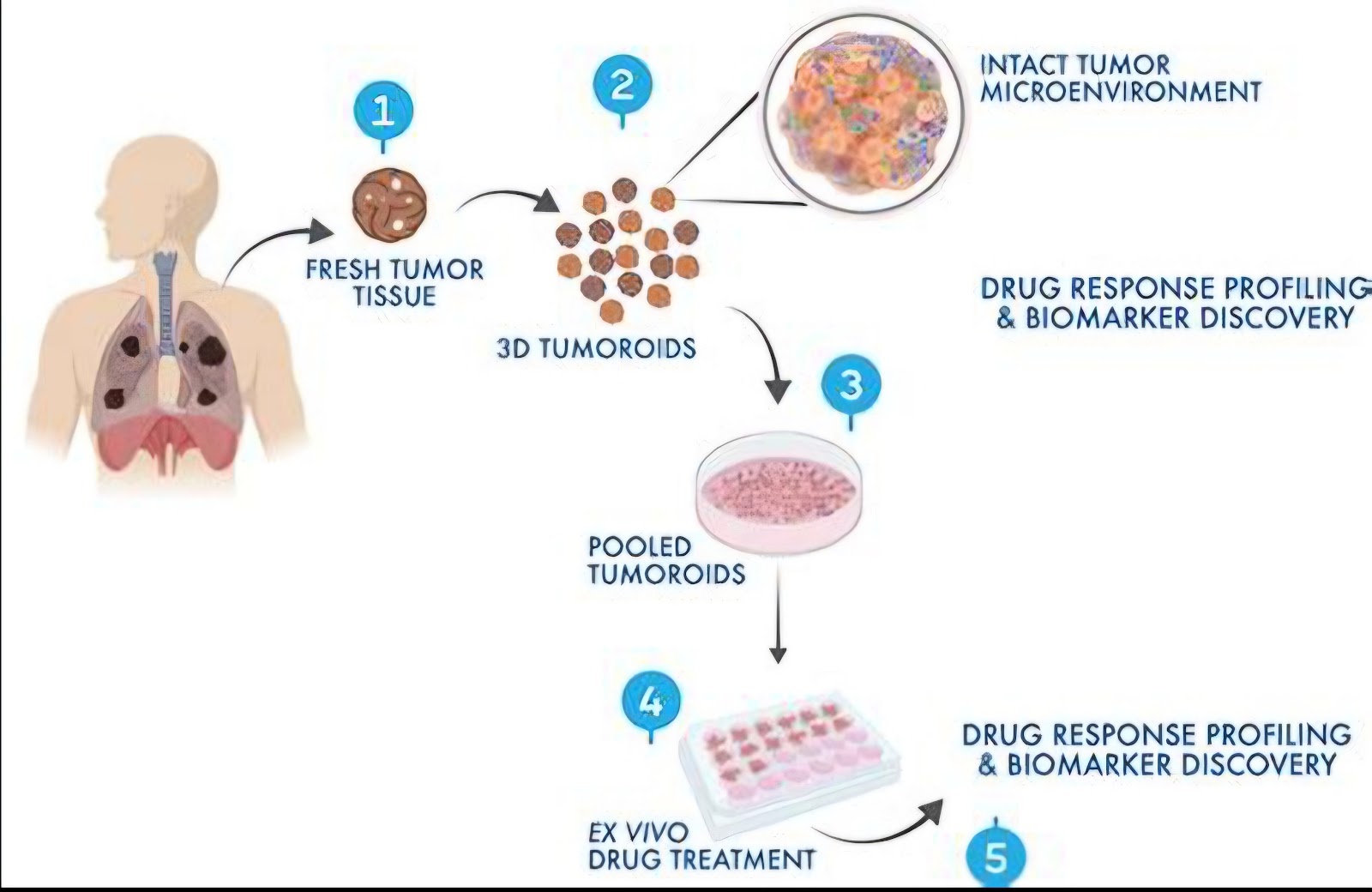
Tumoroid-on-a-Chip technology integrates microfluidics with 3D tumor cultures, allowing precise control over biochemical and biomechanical signals within the tumor microenvironment (TME). These devices simulate the dynamic interactions between cancer cells, stromal cells, and blood vessels, overcoming limitations of traditional 2D cultures and animal models.
1.2 Fabrication and Design
Microfabricated chips contain perfusable microchannels that house patient-derived tumoroids.
Fluidic flow replicates nutrient diffusion, waste removal, and oxygen gradients.
Advanced models integrate vascularized tumoroids with endothelial cells to mimic angiogenesis.
1.3 Applications
Drug screening for personalized medicine.
Studying tumor invasion, metastasis, and immune evasion.
High-throughput screening for radiotherapy and chemotherapy efficacy.
Reference: Nature Communications, 2023, "Microfluidic Tumoroid-on-a-Chip for Precision Oncology."
2. Organoid-Tumoroid Hybrid Models: Bridging Normal and Malignant Biology
2.1 Concept of Organoid-Tumoroid Fusion
Organoids are 3D structures derived from stem cells that replicate organ architecture. Tumoroid-Organoid Hybrid Models fuse normal organoids with tumoroids, enabling the study of cancer progression in a controlled setting.
2.2 Applications
Modeling the transition from pre-malignant to malignant states.
Testing how environmental factors contribute to tumorigenesis.
Developing patient-derived hybrid models for precision oncology.
2.3 Challenges and Future Directions
Standardizing culture conditions for hybrid models.Ensuring genetic stability of long-term cultures.Integrating hybrid models into AI-driven drug screening.
Reference: Cell Stem Cell, 2024, "Organoid-Tumoroid Hybrid Models for Studying Cancer Progression."
3. Tumoroid-Derived Extracellular Vesicles (EVs): A New Frontier in Liquid Biopsy
3.1 What are Tumoroid-Derived EVs?
Extracellular vesicles (EVs) are nanosized membrane-bound vesicles secreted by cells, including tumoroids. They carry proteins, lipids, and nucleic acids, reflecting the molecular profile of the parent tumor.
3.2 Applications of Tumoroid-Derived EVs
Liquid biopsy: Non-invasive cancer diagnosis and monitoring via EVs in blood or urine.
Tumor communication: Studying how tumors manipulate the immune system.
Drug delivery: Engineering tumoroid-derived EVs to transport therapeutic molecules.
3.3 Isolation and Characterization
Ultracentrifugation, size-exclusion chromatography, and microfluidic isolation.
Characterization via nanoparticle tracking analysis (NTA) and electron microscopy.
Reference: Journal of Nanobiotechnology, 2024, "Extracellular Vesicles from Tumoroid Cultures as Next-Generation Biomarkers."
4. Integrating Tumoroids with Immuno-Oncology: Modeling the Tumor-Immune Interface
4.1 Immune-Tumoroid Co-Cultures
The immune system plays a crucial role in cancer progression and therapy. Advanced tumoroid models now incorporate T cells, natural killer (NK) cells, and macrophages to study immune-tumor interactions.
4.2 Applications
Checkpoint inhibitor screening: Evaluating the effectiveness of immunotherapies like anti-PD-1 and anti-CTLA-4.
CAR-T cell therapy testing: Assessing engineered immune cells against patient-derived tumoroids.
Understanding tumor immune evasion: Identifying molecular pathways tumors use to evade immune attacks.
4.3 Advances in AI and Automation
AI-powered imaging and deep learning algorithms are being integrated with these models to analyze immune cell infiltration and drug responses.
Reference: Cancer Immunology Research, 2024, "Organoid-Based Tumor-Immune Models for Immunotherapy Development."
Conclusion: A Converging Future for Cancer Research
The integration of Tumoroid-on-a-Chip, Organoid-Tumoroid Hybrids, EV-based liquid biopsies, and Immuno-Oncology Models represents a major leap in cancer research. These cutting-edge technologies enable personalized and precision oncology, enhancing our understanding of tumor biology while accelerating the development of next-generation therapeutics.
Future Directions
Integration of AI-driven analysis for high-throughput screening.
Hybrid multi-organ-on-a-chip models combining tumoroids with liver, gut, and brain organoids.
Standardized protocols for regulatory approval and clinical translation.
By leveraging these innovative platforms, we are paving the way for breakthroughs in oncology, bringing us closer to highly effective, patient-specific cancer treatments.




Comments
Post a Comment